Anode is Positive or Negative
Cathode - The electrode that receives electrons - positive ions are discharged negative ions are formed. Considering practical implementation it is necessary to use a high areal cathode capacity and limited Na metal namely low negative to positive electrode capacity NP 2 but it is largely neglected in previous work table S7.
Falling positive anode current accompanied by rising anode voltage gives the anode characteristic a region of negative slope and this corresponds to a negative resistance which can cause instability in certain circuits.

. Components of Cells and Batteries. The cathode is protected from corrosion. The positive ions are all attracted to the negative cathode and some pass through the holes in the cathode.
The Anode and Cathode. In a higher range of anode voltage the anode voltage sufficiently exceeds that of the screen for an increasing proportion of the secondary electrons. An anode is a negative electrode and its one of the essential parts of a battery.
As the anode half cell becomes overwhelmed with Cu 2 ions the negative anion of the salt will enter the solution and stabilized the charge. Electrolyte - The conductor through which current is carried. These are the anode rays.
Its usually made of a metal that oxidizes and sends electrons to the cathode the positive electrode. In one form of electroplating the metal to be plated is located at the anode of the circuit with the item to be plated located at the cathode. Thus harsh conditions were explored with cathode mass loading up to 7 mg cm 2 and NP down to 12 fig.
Both the anode and cathode are immersed in a solution that contains a dissolved metal saltsuch as an. By the time they reach the cathode the ions have been accelerated to a sufficient speed such that when they collide with other atoms or molecules in the gas they excite the species to a higher energy level. Similarly in the cathode half cell as the solution becomes more negatively charged cations from.
Return Current Path - The metallic pathway connecting the anode to the cathode. These are the anode rays. This is an electrochemical reaction that produces electrons ie electricity.
The Anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during and electrochemical reaction. The Cathode is the positive or oxidizing electrode that acquires electrons from the external circuit and is reduced during the electrochemical reaction. When they reach the positive anode they transfer their electrons to it and lose their negative charge.
In order to balance the charge on both sides of the cell the half cells are connected by a salt bridge. Electrolytes include aqueous solutions or other liquids. Prepare cells with different electrodes and concentrations and measure their voltages.
Cells are comprised of 3 essential components.

Anodes And Cathodes Positive And Negative Bar Chart Mcat

How To Find Anode And Cathode In Diode Diode Black Rings Tutorial

Electrophoresis Chemistry Anode Cathode Diagram Chemistry Respiratory Therapy Study Materials
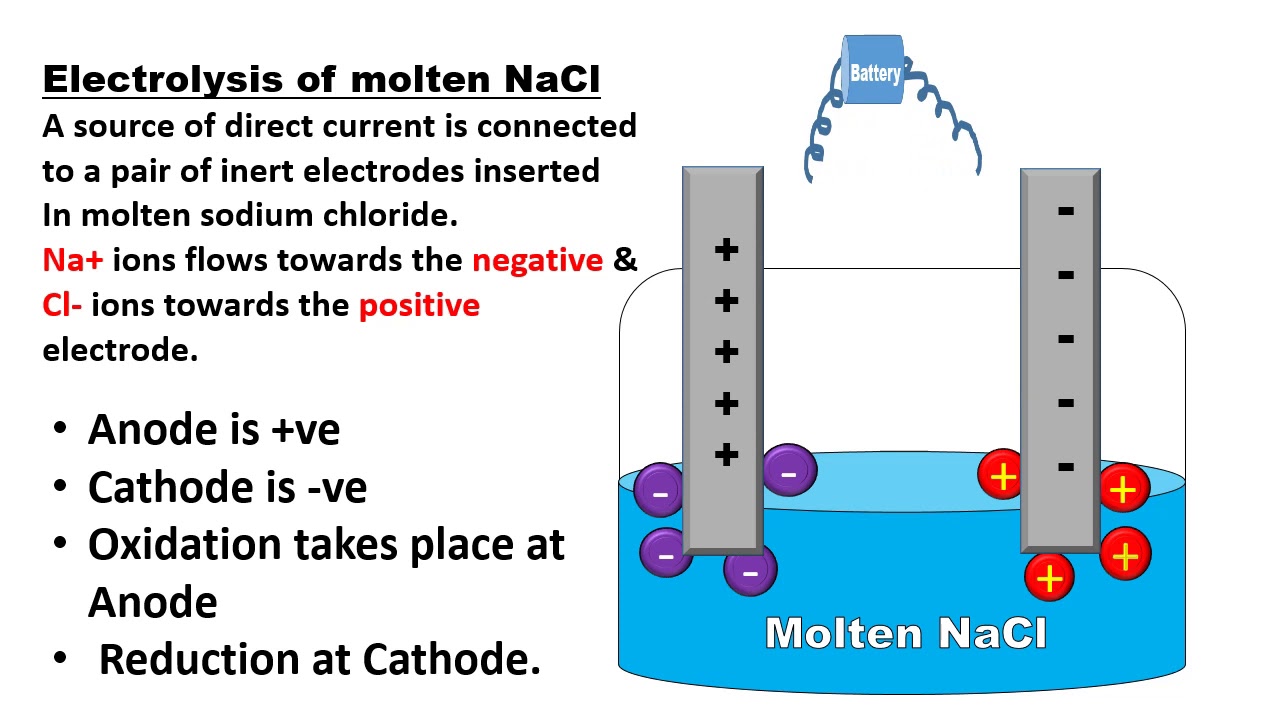
What Is Electrolytic Cell Electrochemistry Chemistry Basics Chemistry

No comments for "Anode is Positive or Negative"
Post a Comment